
Inspire Upper Airway Stimulation - P130008
This is a brief overview of information related to FDA's approval to market this product. See the links below to the Summary of Safety and Effectiveness Data (SSED) and product labeling for more complete information on this product, its indications for use, and the basis for FDA's approval.
Product Name: Inspire® Upper Airway Stimulation (UAS)
PMA Applicant: Inspire Medical Systems, Inc.
Address: 9700 63rd Avenue North, Suite 200
Maple Grove, MN 55369
Approval Date: April 30, 2014
Approval Letter: Not Yet Available
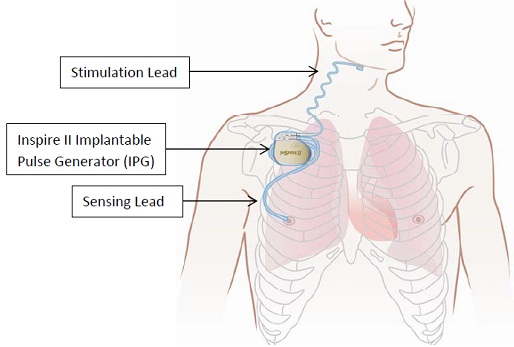
What is it? The Inspire Upper Airway Stimulation (UAS) system is an implantable nerve stimulator used to treat moderate to severe obstructive sleep apnea1 (OSA). The Inspire UAS system consists of implanted components including the implantable pulse generator (IPG), stimulation lead, and sensing lead and external components including the physician programmer and the patient programmer (sleep remote).
How does it work? The IPG detects the patient's breathing pattern and maintains an open airway with mild stimulation of the hypoglossal nerve, which controls tongue movement, during inhaled breathing. The physician adjusts the stimulation settings using the external physician programmer. The patient sleep remote allows the patient to turn therapy on before they go to sleep and to turn therapy off when they wake up.
When is it used? The Inspire UAS system is used to treat a subset of patients with moderate to severe OSA (apnea-hypopnea index [AHI] of greater or equal to 20 and less than or equal to 65). The Inspire UAS system is used in adult patients 22 years and older who have been confirmed to fail or cannot tolerate positive airway pressure (PAP) treatments (such as continuous positive airway pressure [CPAP] or bi-level positive airway pressure [BPAP] machines) and who do not have a complete concentric collapse (as seen during drug induced sleep endoscopy) at the soft palate level.
PAP failure is defined as an inability to eliminate OSA (AHI of greater than 20 despite PAP usage) and PAP intolerance is defined as:
- Inability to use PAP (greater than 5 nights per week of usage; usage defined as greater than 4 hours of use per night), or
- Unwillingness to use PAP (for example, a patient returns the PAP system after attempting to use it).
What will it accomplish? One hundred twenty-six (126) patients participated in a clinical study in 22 different sites. The Inspire UAS therapy provided the majority of patients with significant reductions in the severity of their obstructive sleep apnea and improvements in their quality of life. More than half of all patients experienced at least a 50% reduction in AHI and an AHI of less than 20 events per hour at the end of the 12 month study. Inspire UAS therapy patients also experienced at least a 25% reduction in oxygen desaturation index.
When should it not be used? The Inspire UAS system is contraindicated for:
- Central + mixed apneas greater than 25% of the total AHI.
- Any anatomical finding that would affect the performance of upper airway stimulation, such as the presence of complete concentric collapse of the soft palate.
- Any condition or procedure that would affect neurological control of the upper airway.
- Patients who are unable or do not have the necessary assistance to operate the sleep remote.
- Patients who are pregnant or plan to become pregnant.
- Patients who will require magnetic resonance imaging (MRI).
- Patients with an implantable device that may have unintended interactions with the Inspire system.
Additional information: The Summary of Safety and Effectiveness Data and labeling will be available online.
